Hudson Giovani Zanin
Professor at the Faculty of Electrical and Computer Engineering at the UNICAMP
OpAA74
Vehicles electrified or electric vehicles
Co-author: Fábio Coutinho Antunes, Postdoctoral student at the Faculty of Electrical and Computer Engineering at Unicamp
Ethanol-fueled, electrified or hybrid combustion vehicles emit less carbon dioxide over their cradle-to-grave lifespan compared to purely electric plug-in vehicles. Therefore, these vehicle options should be preferred by the consumer and precede electric vehicles as they emit less greenhouse gases. After all, the energy transition we are experiencing is about reducing greenhouse gas emissions, a phenomenon of global warming, potentiated by human activities.
These gases accumulate between the stratosphere and the troposphere at 12 kilometers altitude, just below the ozone layer, and prevent the Earth's infrared radiation from being released into outer space, preventing the Earth from cooling naturally and gradually. Since the Industrial Revolution (from 1760 to 1820), we started to emit a greater amount of Greenhouse Gases, a fact recognized by the scientific community. Global warming is a major problem, with reflections of human activities on the climate, with floods, prolonged droughts, heat waves in colder times and vice versa , and the melting of the ice caps at the poles, promoting an increase in the level of oceans.
Today, we have social awareness, technical capacity, political will and, above all, solutions that would generate wealth and jobs for society. From a technological point of view, there is a set of technologies that can help us, in a more intelligent way, in this transformation. In other words, it is important to carry out the energy transition, using the best in each location.
The energy transition will be unique to each region of the world. In Brazil, we have ethanol, a low-carbon biofuel. The ethanol vehicle emits carbon monoxide and hydrocarbons on its cold start. Hybrid vehicles, with two engines, brought the advantage of reducing carbon emissions, in addition to greater autonomy. But it can get better.
One of the solutions is to use a hybrid powertrain system of lower complexity and cost for on-board electrical power generation in the mobility sector. This system is composed of an ethanol reformer in synthesis gas (Molecular Hydrogen and Carbon Monoxide). This gas is converted directly into electrical energy by a stack of solid oxide fuel cells. The electrical energy generated supplies power to a high-efficiency electric motor ( 95% efficiency ), while electrically charging a much smaller module of lithium-ion batteries. This entire system can achieve up to 70% efficiency. Much higher than the 35% maximum efficiency of an internal combustion vehicle.
This solution takes advantage of the value network created years ago by ethanol generation , logistics and distribution at gas stations. And most importantly, filling with a mixture of 55% by volume of ethanol and 45% by volume of water in 5 minutes. Water is important for the efficient reforming of ethanol, producing much more molecular hydrogen. In addition, the cost of fuel to the consumer is expected to fall to half the current value, and the range must be compatible with current vehicles. Other than that, the carbon emission will be much more reduced.
We have not yet fully mastered solid oxide fuel cell technology to the point of bringing it to market. It is already used to generate stationary energy with natural gas and pure molecular hydrogen, but it needs to be adapted for the synthesis gas of reformed ethanol and applied in mobility. The stack weight of these fuel cells needs to be reduced, and efficiency improved, which should happen in the next 5 years.
The plug-in vehicle, on the other hand, is ready, and carries the promise of zero emissions by not emitting Greenhouse Gases. But this story is not so realistic. In their life cycle, they need to travel approximately 100,000 kilometers to match the carbon dioxide emissions of a combustion vehicle fueled with ethanol, that is, practically its entire useful life. In addition, the cost, replacement of battery modules and maintenance are quite high.
There are many reports of damage to batteries due to road imperfections, problems in the charging system, need for investment in infrastructure for the distribution and refueling network. The cost of generating clean and renewable electricity, distribution (networks) and charging stations for plug-in vehicles in Brazil is estimated at 300 billion dollars. This investment in infrastructure could vastly benefit the energy generation, transmission and distribution chain, and the bill should not be placed solely on the electric vehicle. It also makes no sense to have a vehicle that plugs and charges it using electrical energy generated by thermoelectric plants.
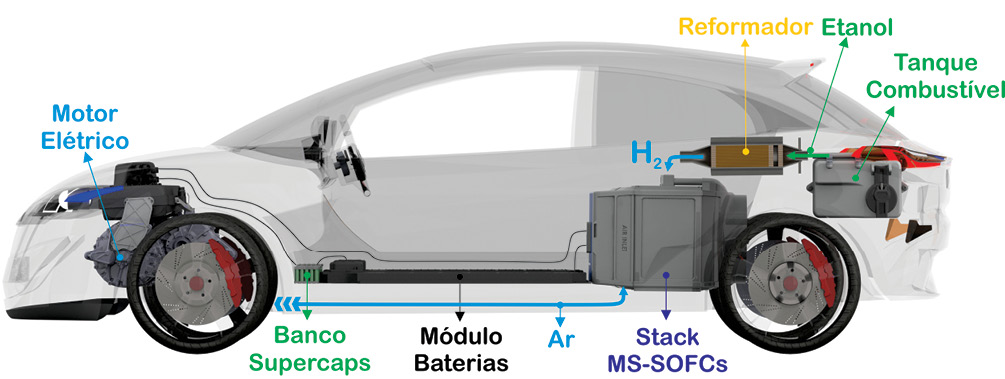
The subject is complex and requires reflection for better clarification. The following is a brief discussion of the main features of this ethanol-powered hybrid powertrain system, presented below. The following is a breakdown of the reformer, solid oxide fuel cell, supercapacitors, batteries and electric motor.
Reformers:
The ethanol reformer is similar to the catalysts in a combustion vehicle. The reformer is composed of a monolithic ceramic support obtained by extrusion or metallic, with fine channels of the unidirectional or honeycomb type. The channels are coated with a second ceramic bonding layer composed of materials with high oxygen storage and release capacity.
On this layer, several catalyst active metal nanoparticles supported on (or even inside) porous ceramic nanoparticles obtained by impregnation, solvothermal infiltration or coprecipitation of precursor salt solutions. After a drying and calcination process, the active phases of the catalysts with high dispersion and surface area are obtained. These catalysts are responsible for converting ethanol into synthesis gas with high selectivity of molecular Hydrogen at a temperature of approximately 650 degrees centigrade.
State-of-the-art materials used in ethanol reformers are:
1) monolithic cordierite ceramic supports , with low coefficient of thermal expansion (5 times 10 minus 6 meters per degree celsius) and high resistance to thermal shock or metallic supports in stainless steel ferritic or austenitic of high thermal conductivity and resistance to thermal shock;
2) a ceramic adhesion layer of Cerium Oxide, Cerium Oxide Zirconia Oxide of high capacity for oxygen storage and release, or even Aluminum Oxide or Zirconia Oxide, doped with Magnesium Oxide to improve the storage capacity and oxygen release; and
3) nanoparticles of catalyst metals such as Nickel, Nickel Cobalt, Nickel Molybdenum, Nickel Niobium, Nickel Copper, supported on ceramic nanoparticles of the same materials as the ceramic adhesion layer. The reformed synthesis gas from Molecular Hydrogen-rich ethanol is directed to the solid oxide fuel cell stack to be converted by the cell anode directly into electrical energy, heat, water and carbon dioxide.
Solid Oxide Fuel Cells:
A fuel cell is a solid state electrochemical device that converts the energy of chemical bonds in a fuel into electrical energy, similar to batteries in that electrochemical reactions at the electrodes generate electricity. In solid oxide fuel cells, the molecular oxygen present in the air is reduced at the cathode by electrons, forming oxygen anions . These oxygen anions pass through the electrolyte towards the anode to oxidize Molecular Hydrogen releasing electrons to the external circuit and then these electrons back to the cathode to reduce the oxygen from the air into oxygen anions. This electrochemical reaction not only produces electrons, but also heat and water.
We cannot use direct molecular hydrogen because it is a gas that needs to be cryoliquefied and stored in high-cost tanks, with risks of explosion in the event of a collision. Hydrogen, on the other hand, is an element present in hydrocarbon molecules and biofuels, such as ethanol. Ethanol has 6 hydrogen atoms that can form 3 molecules of molecular hydrogen (Ethanol). That is, biofuels store hydrogen in liquid form. Ethanol stores 18.4 Mega Joules per liter, while molecular Hydrogen stores only 0.01 Mega Joules per liter of energy under Normal Temperature and Pressure Conditions.
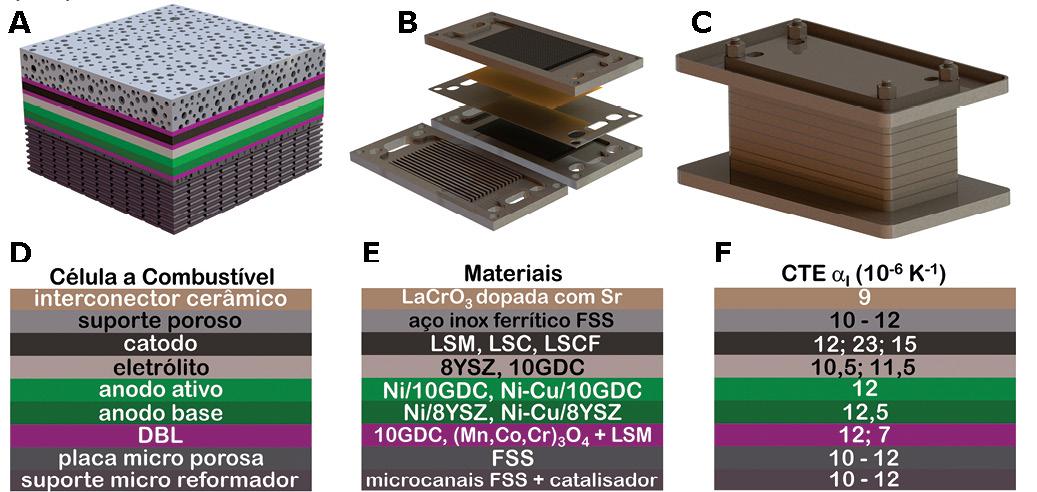
The featured figure presents a schematic of the solid oxide fuel cell with the components, state-of-the-art materials and the respective thermal expansion coefficients. In addition to the solid oxide fuel cell, we can see their stack in the figure. The system produces electricity to power the engine and batteries. In cases of peak power demand, such as overtaking, the battery module or a bank of supercapacitors can quickly supply power to the electric motor.
The seat is used to quickly reabsorb regenerative energy from the electric motor during vehicle deceleration or braking. In this configuration, the lithium-ion battery module suppresses the inherent power fluctuation of the solid oxide fuel cell stack. The figure shows:
A: Computer Aided Design enlargement of a cross-section of a unit cell Metal support solid oxide fuel cell and its components,
B: Computer Aided Design of a unit cell Metal support fuel cell of solid oxide and its interconnectors,
C: Computer Aided Design of a Solid Oxide Fuel Cell Metal Support Stack,
D: Solid Oxide Fuel Cell Metal Support Components,
E: Materials Used in the Metal Support Components solid oxide fuel cells,
F: coefficients of thermal expansion of component materials used in metal support solid oxide fuel cells.
Alternating current asynchronous and magnetic electric motors exhibit high torque, efficiency in converting electrical energy into kinetics and excellent rotational control. Tesla and Honda plug-in vehicle engines have efficiencies of up to 92% and 97%, respectively.
This hybrid powertrain system has the following advantages:
1) small tank to be filled with hydrated ethanol;
2) autonomy depends on the external reformer, the amount of fuel and the efficiencies of the reformer and solid oxide fuel cell stack and not the battery module;
3) the battery module can be reduced by up to a fifth in volume, reducing weight and cost, and
4) the amount of toxic and flammable substances present in the electrodes and electrolytes of lithium-ion batteries will be smaller, also allowing the reduction by demand for Nickel, Lithium, Cobalt, Aluminum, Copper, Silicon, Zinc, Manganese and carbon, often subject to price fluctuations and crises. The Brazilian solution can benefit 2 billion people on the planet for this and future generations.